The standard book on HSP science is Dr Hansen's Hansen Solubility Parameters: A User's Handbook, Second Edition published in 2007 by CRC Press.
- Barton Handbook Of Solubility Parameters 2
- Barton Handbook Of Solubility Parameters Class
- Barton Handbook Of Solubility Parameters Worksheet
It is an official CRC Bestseller!
The Table of Contents gives a good idea of the vast scope of the book and its authoritative nature.
Table of Contents
The Hildebrand solubility parameter (δ) provides a numerical estimate of the degree of interaction between materials and can be a good indication of solubility, particularly for nonpolar materials such as many polymers. Materials with similar values of δ are likely to be miscible. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, Second Edition by Allan F.M. Barton, 766, available at Book Depository with free delivery worldwide.
- 'The CRC Handbook of Solubility Parameters and Other Cohesion Parameters, Second Edition', which includes 17 new sections and 40 new data tables, incorporates information from a vast amount of material published over the last ten years. The volume is based on a bibliography of 2,900 reports, including 1,200 new citations.
- Book Description Now available for the first time, this valuable reference presents polymer solubility parameters and various polymer-liquid interaction parameters in an easy-to-use form. It critically evaluates and comprehensively compiles data from original sources.
- Solubility Parameters — An Introduction;C.M. Hansen
- Hildebrand Parameters and Basic Polymer Solution Thermodynamics
- Hansen Solubility Parameters
- Methods and Problems in the Determination of Partial Solubility Parameters
- Calculation of the Dispersion Solubility Parameter δd
- Calculation of the Polar Solubility Parameter δp
- Calculation of the Hydrogen Bonding Solubility Parameter δh
- Supplementary Calculations And Procedures
- Hansen Solubility Parameters for Water
- Theory — The Prigogine Corresponding States Theory, the c12 Interaction Parameter, and the Hansen Solubility Parameters;C.M. Hansen
- Hansen Solubility Parameters (HSP)
- Resemblance Between Predictions of Hansen Solubility Parameters and Corresponding States Theories
- The c12Parameter and Hansen Solubility Parameters
- Comparison of Calculated and Experimental c12 Parameters
- General Discussion
- Postscript
- Statistical Thermodynamic Calculations of the Hydrogen Bonding, Dipolar, and Dispersion Solubility Parameters;C. Panayiotou
- Theory
- Applications
- Discussion and Conclusions
- Appendix I: The Acid Dimerization
- Appendix II: An Alternative Form of the Polar Term
- Appendix III: A Group-Contribution Method for the Prediction of δ and δD
- Hansen Solubility Parameters (HSP) in Thermodynamic Models for Polymer Solutions;G.M. Kontogeorgis
- Group Contribution Methods for Estimating Properties of Polymers
- Activity Coefficients Models Using the HSP
- Conclusions and Future Challenges
- Appendix I: An Expression of the FH Model for Multicomponent Mixture
- Methods of Characterization — Polymers;C.M. Hansen
- Calculation of Polymer HSP
- Solubility — Examples
- Swelling — Examples
- Melting Point Determinations — Effect of Temperature
- Environmental Stress Cracking
- Intrinsic Viscosity Measurements
- Other Measurement Techniques
- Methods of Characterization — Surfaces;C.M. Hansen
- Hansen Solubility Parameter Correlations with Surface Tension (Surface Free Energy)
- Method to Evaluate the Cohesion Energy Parameters for Surfaces
- A Critical View of the Critical Surface Tensions
- A Critical View of the Wetting Tension
- Additional Hansen Solubility Parameter Surface Characterizations and Comparisons
- Self-Stratifying Coatings
- Maximizing Physical Adhesion
- Methods of Characterization for Pigments, Fillers, and Fibers;C.M. Hansen
- Methods to Characterize Pigment, Filler, and Fiber Surfaces
- Discussion — Pigments, Fillers, and Fibers
- Hansen Solubility Parameter Correlation of Zeta Potential for Blanc Fixe
- Carbon Fiber Surface Characterization
- Controlled Adsorption (Self-Assembly)
- Applications — Coatings and Other Filled Polymer Systems;C.M. Hansen
- Solvents
- Techniques for Data Treatment
- Solvents and Surface Phenomena in Coatings (Self-Assembly)
- Polymer Compatibility
- Hansen Solubility Parameter Principles Applied to Understanding Other Filled Polymer Systems
- Hansen Solubility Parameters of Asphalt, Bitumen and Crude Oils;P. Redelius
- Models of Bitumen
- Asphaltenes
- Molecular Weight
- Polarity
- Solubility Parameters of Bitumen
- Testing of Bitumen Solubility
- Hildebrand Solubility Parameters
- Hansen Solubility Parameters (HSP)
- The Solubility Sphere
- Computer Program for Calculation and Plotting of the Hansen 3D Pseudosphere
- Components of Bitumen
- Bitumen and Polymers
- Crude Oil
- Turbidimetric Titrations
- BISOM Test
- Determination of Hansen Solubility Parameter Values for Carbon Dioxide;L.L. Williams
- Methodology
- One-Component Hildebrand Parameter as a Function of Temperature and Pressure
- Three-Component (Hansen) Solubility Parameters — Pure CO2
- Temperature and Pressure Effects on HSPs: δd
- Temperature and Pressure Effects on HSPs: δp
- Temperature and Pressure Effects on HSPs: δh
- Addendum
- Appendix I: Ideal Solubility of Gases in Liquids and Published CO2 Solubility Data
- Use of Hansen Solubility Parameters to Identify Cleaning Applications for “Designer” Solvents;J. Durkee
- A Variety of Solvents
- Pathology of Soils
- HSP of Multiple-Component Soils
- Method for Calculating HSP of Composites (Soils or Solvents)
- More Realistic View About Evaluating HSP of Composite Soils
- Method for Choice of Suitable Solvents
- Reference Soils for Comparison
- Identification of Designer Solvents
- An Open Question — Answered
- Limiting RA Value For Expected Good Cleaning Performance
- Application of HSP Methodology to Cleaning Operations
- Analysis of Capability of Designer Solvents
- Applications — Chemical Resistance;C.M. Hansen
- Chemical Resistance — Acceptable-or-Not Data
- Effects of Solvent Molecular Size
- Chemical Resistance — Examples
- Special Effects with Water
- Applications — Barrier Polymers;C.M. Hansen
- Concentration-Dependent Diffusion
- Solubility Parameter Correlations Based on Permeation Phenomena
- Solubility Parameter Correlation of Polymer Swelling
- Solubility Parameter Correlation of Permeation Coefficients for Gases
- General Considerations
- Applications – Environmental Stress Cracking in Polymers;C.M. Hansen
- ESC Interpreted Using HSP
- ESC With Nonabsorbing Stress Cracking Initiators
- Hansen Solubility Parameters — Biological Materials;C.M. Hansen and T. Svenstrup Poulsen
- Hydrophobic Bonding and Hydrophilic Bonding (Self-Association)
- DNA
- Cholesterol
- Lard
- Human Skin
- Proteins — Blood Serum and Zein
- Chlorophyll and Lignin
- Wood Chemicals and Polymers
- Urea
- Water
- Surface Mobility
- Chiral Rotation, Hydrogen Bonding, and Nanoengineering
- Absorption and Diffusion in Polymers;C.M. Hansen
- Steady State Permeation
- The Diffusion Equation
- Surface Resistance
- Side Effects
- Film Formation by Solvent Evaporation
- Anomalous Diffusion (Case II, Super Case II)
- Applications — Safety and Environment;C.M. Hansen
- Substitution
- Alternative Systems
- Solvent Formulation And Personal Protection For Least Risk
- The Danish Mal System — The Fan
- Selection of Chemical Protective Clothing
- Uptake of Contents by a Plastic Container
- Skin Penetration
- Transport Phenomena
- The Future
- Hansen Solubility Parameter Data and Data Quality
- Group Contribution Methods
- Polymers as Points — Solvents as Spheres
- Characterizing Surfaces
- Materials and Processes Suggested for Further Attention
- Theoretical Problems Awaiting Future Resolution
- Appendices
- Hansen Solubility Parameters for Selected Solvents with the major contribution of Hanno Priebe
- Hansen Solubility Parameters for Selected Correlations
- Solubility Data for the Original 33 Polymers and 88 Solvents
- Index
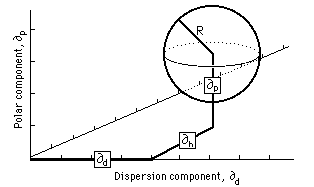
Hansen Solubility Sphere
Barton Handbook Of Solubility Parameters 2
Hansen solubility parameters were developed by Charles M. Hansen in 1967 to predict the solubility of polymers in solvents. They are widely used in the paint and coatings industry. The method is based on the idea that like dissolves like. This is the case when the solvent and the solute have similar Hansen Solubility Parameters.
The three Hansen parameters can be considered the coordinates of a point in the so-called Hansen space. The closer two points are, the more likely the compounds are to dissolve into each other. In the case of a polymer, only solvents within a certain range will dissolve the polymer. This range is usually an ellipsoid and only solvents within this space are likely to dissolve the polymer in question.
Barton Handbook Of Solubility Parameters Class
Hansen Solubility Sphere
Occasionally, the scale of the dispersion axis is doubled, providing approximately a spherical volume of solubility. Then the distance of the solvent coordinates (δd2, δp2, δh2) from the center point (δd1, δp1, δh1) of the solute sphere is given by
Ra2 = 4(δd2 - δd1)2 + (δp2 - δp1)2 + (δh2 - δh1)2
The distance Ra in the equation above can be compared with the solubility radius of the polymer, R0. If Ra < R0 then there is a high likelihood of the solvent to dissolve the polymer. The radius of the solubility sphere is often called the interaction radius and the ratio Ra / R0 the relative energy difference (RED) of the system.
Ra / R0 > 1 → the compound is a non-solvent
Barton Handbook Of Solubility Parameters Worksheet

Ra / R0 < 1 → the compound is a solvent
Ra / R0 = 0 → the compound may cause swelling
Hansen1 and Barton2 have tabulated the interaction radius and partial solubility parameters for many polymers and solvents.
To give an example, we calculate Ra / R0 of polystyrene (PS) and acetone. According to Hansen1, PS has an interaction radius of approximately 12.7 and its partial solubility parameters are (δd, δp, δh) = (21.3, 5.7, 4.3) and those of acetone are (15.5, 10.4, 7.0). This gives
Ra = {(2·15.5 - 2·22.3)2 + (10.4 - 5.8)2 + (7.0 - 4.3)2}1/2 = 12.8
→ Ra / R0 = 12.8 / 12.7 ≈ 1.0

This result indicates that acetone is not a solvent for polystyrene but might cause some swelling of the polymer.
| Compound | δp | δd | δh |
| Poly(vinylchloride), PVC | 8.8 | 18.6 | 5.8 |
| Polychloroprene, Neoprene | 4.3 | 19.5 | 3.1 |
| Polyethylene, PE | 0.0 | 17.6 | 0.0 |
| Poly(isobutylene) | 2.5 | 16.2 | 4.3 |
| Polypropylene | 0.0 | 18.0 | 0.0 |
| Nylon 6,6 | 5.1 | 18.2 | 13.7 |
| Poly(1,4-butadiene) | 2.3 | 17.3 | 2.6 |
| Polyisoprene | 1.1 | 16.9 | -0.4 |
| Poly(ethylene terephthalate), PET | 7.3 | 18.2 | 7.9 |
| Poly(ethyl methacrylate), PEMA | 7.8 | 17.9 | 3.4 |
| Poly(methacrylic acid) | 12.5 | 17.4 | 16.0 |
| Poly(methyl methacrylate), PMMA | 10.5 | 18.8 | 5.7 |
| Poly(acrylonitrile), PAN | 15.1 | 20.0 | 7.9 |
| Polystyrene, PS | 5.9 | 18.7 | 3.5 |
| Polysulfone | 8.8 | 18.7 | 6.1 |
| Poly(vinyl alcohol), PVOH | 12.5 | 17.5 | 10.0 |
| Poly(vinyl acetate) | 11.3 | 20.9 | 9.7 |
| Poly(vinyl butyrate), PVB | 4.4 | 18.6 | 13.0 |
| Poly(vinyl butyral) | 9.5 | 19.1 | 8.0 |
| Poly(tetrafluoroethylene), PTFE | 0.0 | 14.0 | 0.0 |
References
- Charles M. Hansen, Hansen Solubility Parameters: A User's Handbook, 2nd Edition, 2007
- Allan F.M. Barton, CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed., 1991